How to download EUDRA data on drug safety surveillance
Learn how to access the European Medicines Agency's (EMA) European Union Drug Safety Surveillance (EUDRA) data through quarterly reports and APIs for post-market drug surveillance.
EudraVigilance is the European Union’s centralized system for managing and analyzing information about suspected adverse reactions to medicines. These medicines can either be authorized for use or still under investigation in clinical trials within the European Economic Area (EEA). The system is operated by the European Medicines Agency (EMA) on behalf of the EU medicines regulatory network. It plays a key role in supporting public health by enabling the monitoring of the safety of medicines and ensuring that regulatory authorities can respond quickly to emerging safety issues.
For more details on EudraVigilance, visit the official page: EudraVigilance Overview.
To learn how to access and download EUDRA data, keep reading.
Accessing EudraVigilance Data
EudraVigilance offers access of adverse reaction reports based on stakeholder groups:
- Marketing authorization holders
- EEA national medicines regulatory authorities
- Academia
- Healthcare professionals, patients and the general public
- World Health Organization - Uppsala Monitoring Centre (WHO-UMC)
- Medicines regulatory authorities outside the EU
More details can be found at the official EudraVigilance Data Access page.
This allows patients, healthcare professionals, and researchers to explore aggregated data on suspected adverse reactions reported across the EEA. The public version contains anonymized data to protect patient confidentiality while maintaining transparency in medicine safety reporting.
EudraVigilance, the publicly accessible European database of suspected adverse drug reaction reports, allows users to search and analyze adverse reaction reports linked to:
- Products: Allows users to search and analyze adverse reaction reports linked to specific medicinal products.
- Substances: Enables access to reports related to active substances, often showing a broader picture of drug reactions across different formulations.
Example: Fentanyl
Dashboard
One example of how EudraVigilance can be used is by analyzing reports related to Fentanyl, a potent opioid used for pain management. Reports for Fentanyl show suspected adverse reactions across different products and highlight the safety concerns related to its use.
- Navigate to the EudraVigilance Public Reports.
- Click on the “Search” button.
- Search by “Substances”.
- Select “F”, the initial letter of the substance.
- Select “Fentanyl” from the list of substances.
You’ll see the following page:
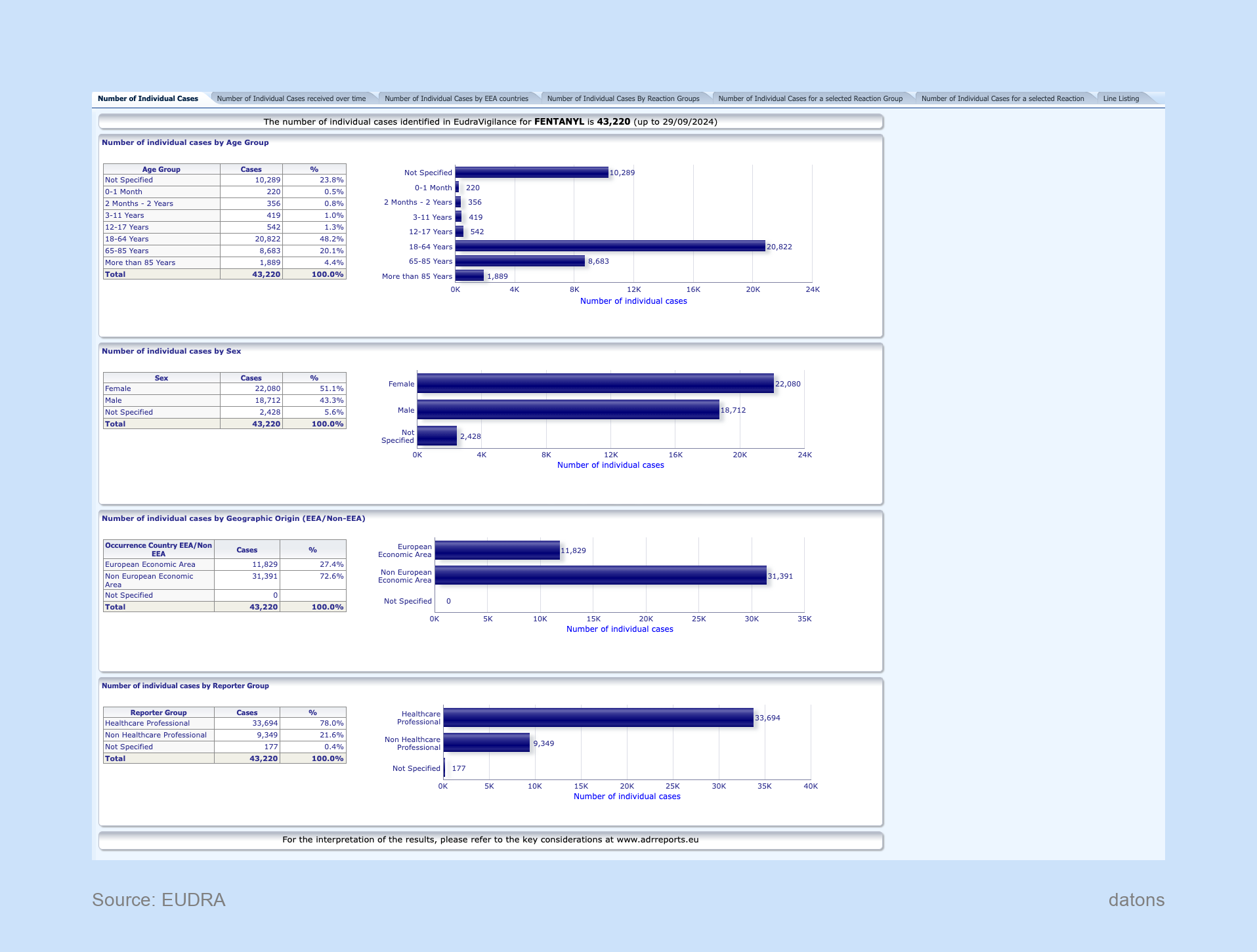
This section gives access to charts, graphs, and detailed data to help users visualize the adverse event trends over time, categorized by severity, demographics, and clinical outcomes.
Downloading Data from EudraVigilance
It’s not trivial to download data from EudraVigilance. After selecting the substance (or product), you navigate to Line Listing section.

You can’t download all data at once, but you can download them by year. Therefore, select the year on Gateway Date and click on Run Line Listing Report.
After a few seconds, scroll down to the bottom and click on Export to get the data file.

Conclusion
EudraVigilance plays an integral role in pharmacovigilance across the EU, helping to ensure medicine safety by tracking adverse reactions. By making this data publicly accessible, it supports transparency in healthcare, allowing researchers and healthcare professionals to monitor and analyze safety concerns in real-time. Whether searching for adverse reaction data related to a specific product or an active substance, EudraVigilance provides a wealth of information that can be accessed online or through downloadable datasets.