FAERS 2024 Q1 Drug Frequency and Adverse Reactions Analysis
An in-depth analysis of the FAERS Q1 2024 data, focusing on drug frequency and adverse reactions.
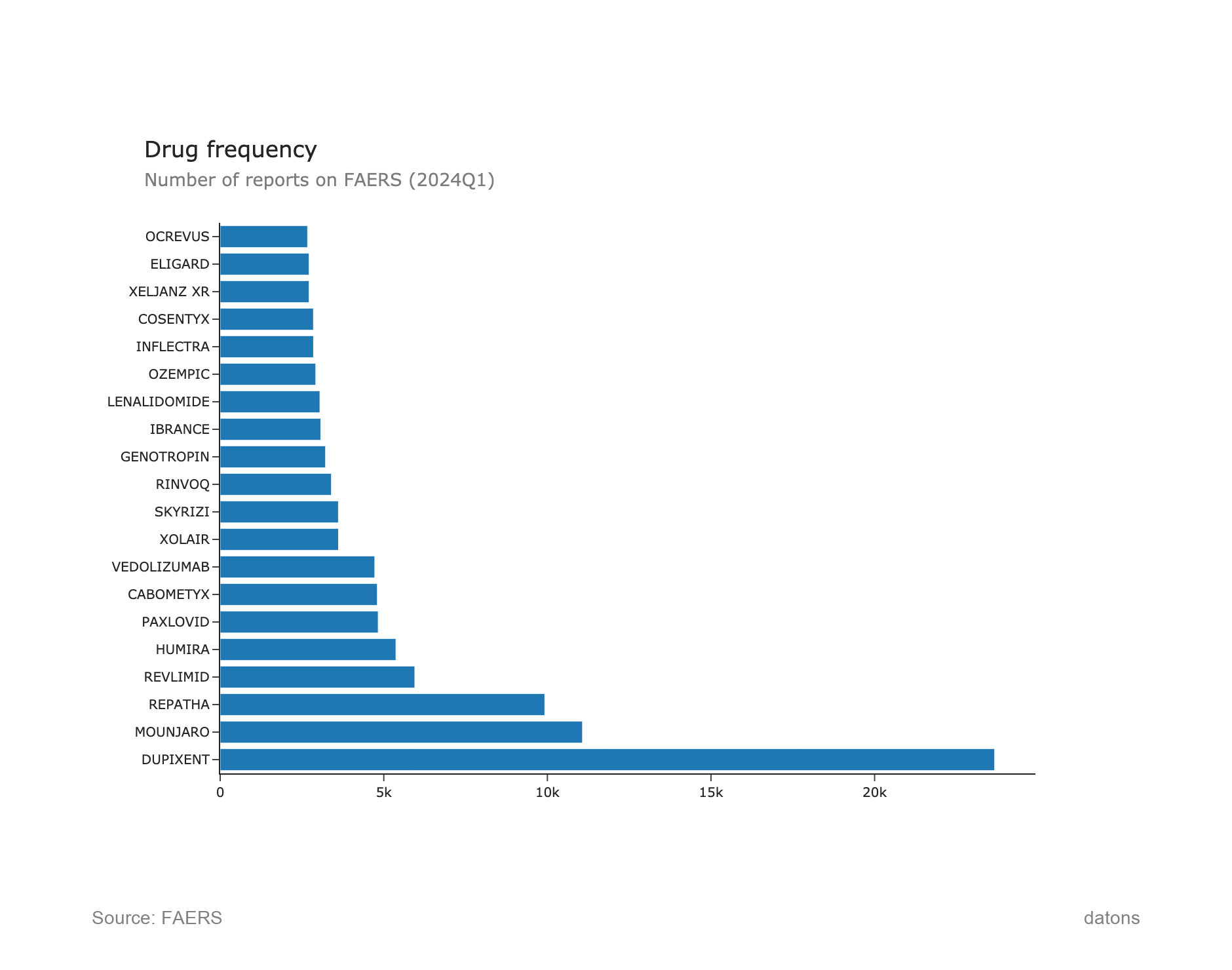
1. Drug Frequency Overview
This chart presents the top 20 drugs by the number of reports on FAERS for Q1 2024. DUPIXENT leads with the highest frequency, followed by MOUNJARO and REPATHA. These drugs have the most reports, indicating higher patient experiences or adverse reactions associated with them.
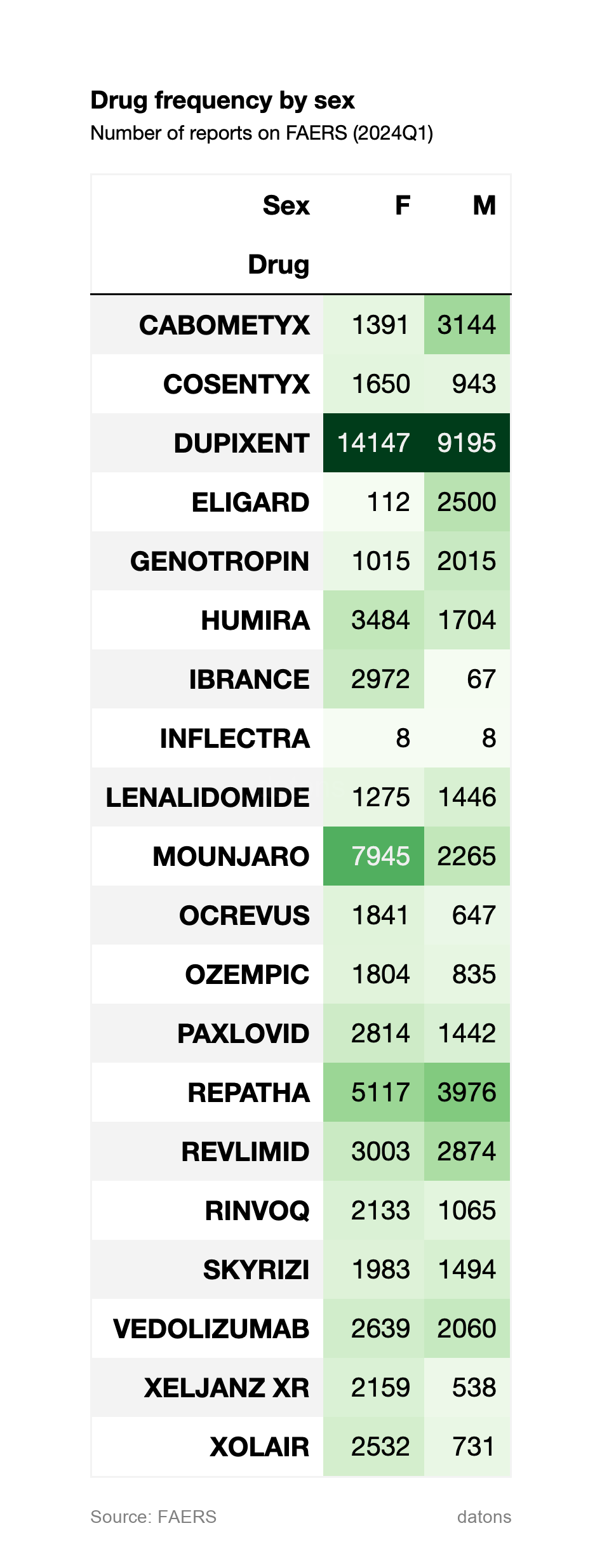
2. Drug Frequency by Sex
The second visualization breaks down the drug frequency by gender. DUPIXENT remains the most reported drug for both females and males, though females show a significantly higher count. ELIGARD and GENOTROPIN show a strong male preference, while HUMIRA and IBRANCE show a higher count in female patients.
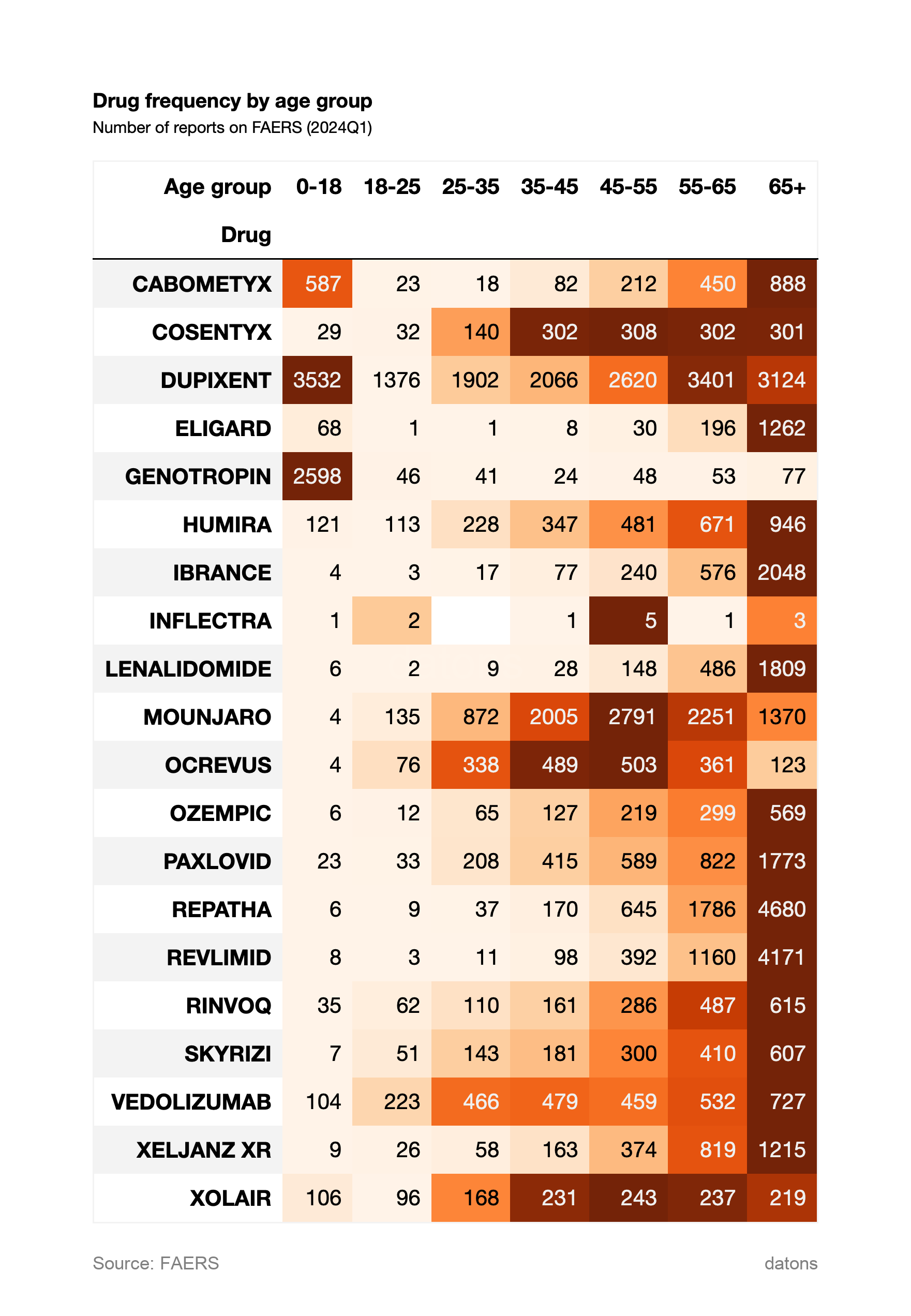
3. Drug Frequency by Age Group
This chart segments drug frequency by age group. DUPIXENT dominates across various age groups, with a higher concentration in patients aged 45-65. GENOTROPIN is more commonly reported for patients aged 0-18, indicating its use in pediatric cases. MOUNJARO shows higher frequency in the 45-65 age range as well.
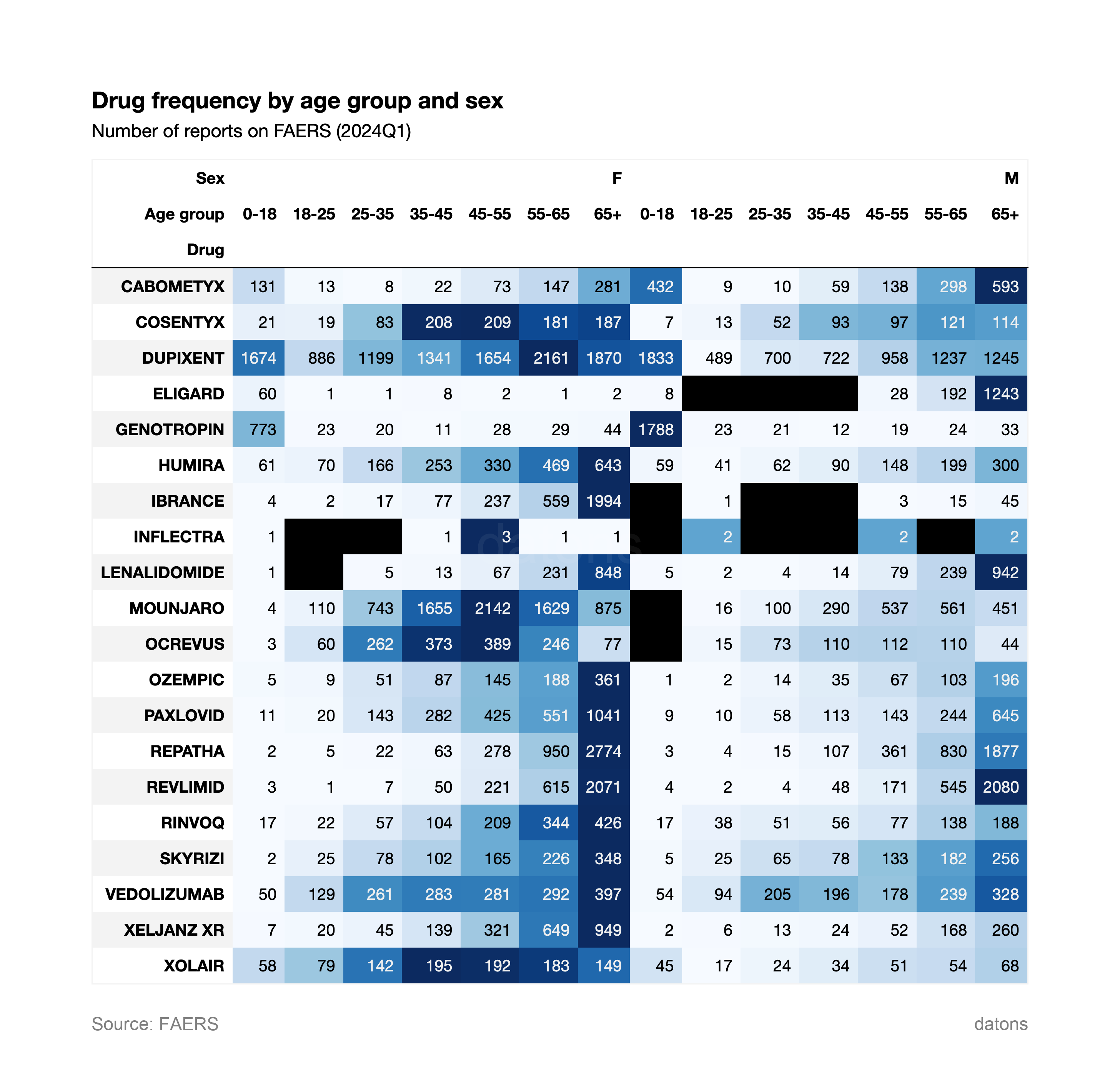
4. Drug Frequency by Age and Sex
This chart combines the frequency of drugs by both age and sex. DUPIXENT is prominent across both genders, with a higher report count in females aged 45-65. ELIGARD shows a strong male prevalence, especially in older age groups (55+). GENOTROPIN shows a higher concentration in younger males (0-18 years old).
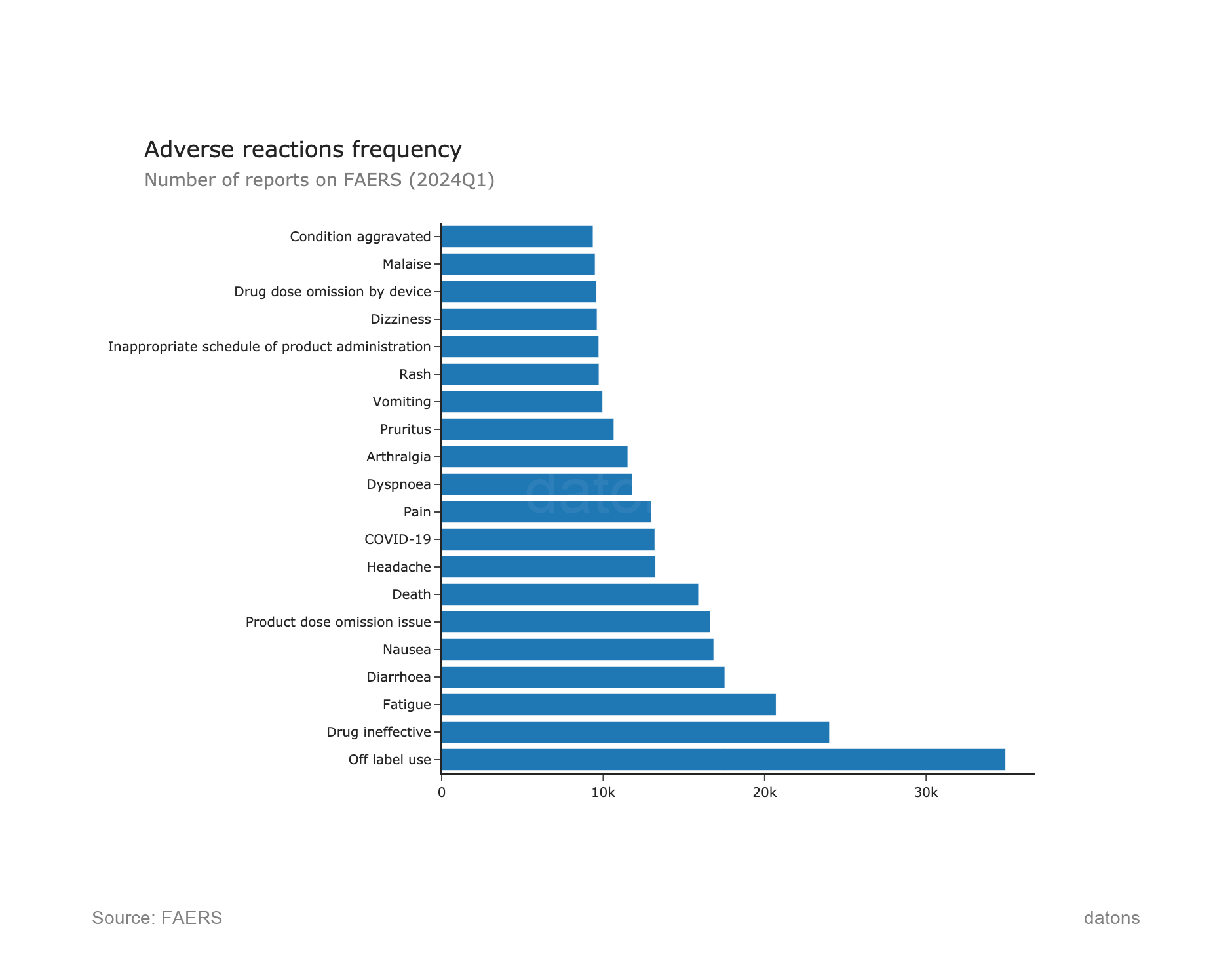
5. Adverse Reactions Frequency
The most common adverse reactions reported in FAERS Q1 2024 include Off-label use, Drug ineffective, and Fatigue. Off-label use stands out with more than 30,000 reports, highlighting its widespread issue in patient treatment.
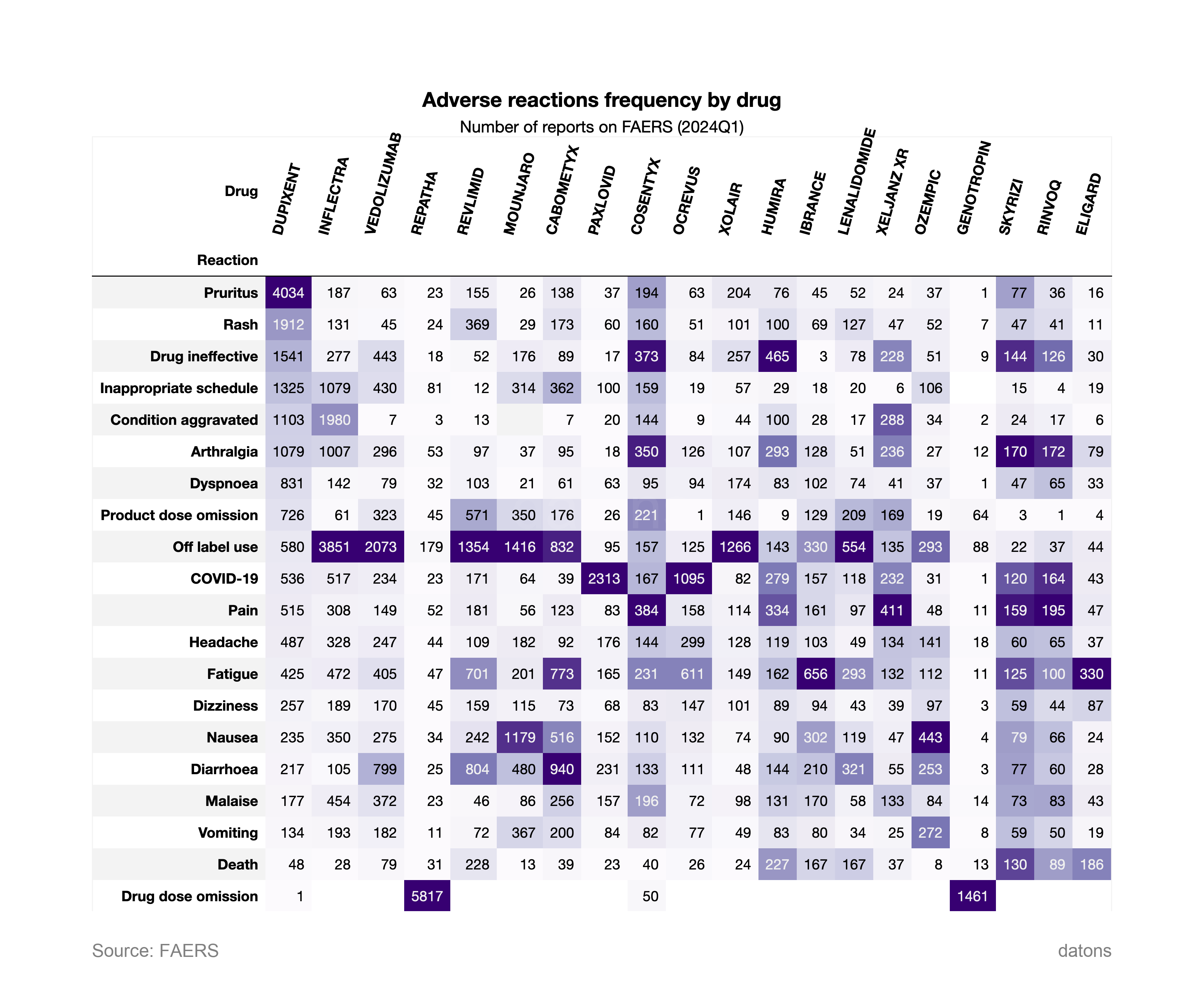
6. Adverse Reactions by Drug
This final chart shows the frequency of adverse reactions by specific drugs. DUPIXENT is strongly associated with Pruritus and Rash, while VEDOLIZUMAB and REPATHA have a higher correlation with Off-label use. This breakdown provides insight into which drugs are linked with certain side effects, crucial for monitoring patient safety.